
OVERVIEW
For many applications, in-situ detection and identification of organic vapors implies the need for a high level of portability and near real-time response. Recently, a hand portable Gas Chromatography/Ion Mobility Spectrometry (GC/IMS) instrument has been demonstrated for detection of toxic chemicals of military concern. This new instrument called the EVM II (Environmental Vapor Monitor), is based upon the combination of Graseby Dynamics' "CAM" (Chemical Agent Monitor) IMS (Ion Mobility Spectrometer) and FemtoScan's "Enviroprobe" Automated Vapor Sampling GC instruments. This powerful combination offers greatly improved interferant rejection and enhanced quantitation when compared to "IMS only" instruments without compromising the portability and rapid response characteristics of portable IMS instruments.
The idea of being able to carry (in only one hand!) a truly powerful, hyphenated analytical instrument is exciting to users with a wide range of applications (.g., everything from an "in-situ" manufacturing process monitor to an on-site environmental monitor to a portable drug or explosive detector). Customer interest has led to continuing efforts to demonstrate the EVM II's specific capability to address various application needs. This note provides an basic overview of the capabilities of the unit for field and process analysis on a broad variety of chemical species.
In addition, the advantages obtained from the integration of FemtoScan's AVS-GC (automated vapor sampling gas chromatography) with IMS technology are discussed. This includes not only the interferant rejection capabilities already mentioned, but also the observation that these hand held, low temperature GC/IMS instruments can operate with lower water vapor concentrations than the membrane introduction IMS devices from which they were developed. This combination opens up the possibility of a number of interesting portable GC/IMS applications.
An examination of a number (~100) of different chemical compounds with a broad range of chemical functionalities under a range of analytical conditions was performed with the instrument in order to evaluate the detection capabilities of the EVM II. This paper will report on preliminary results from this effort to evaluate both the operating capabilities of this new instrument and the analytical performance of the instrument on volatile organic chemical species ranging from polar to non-polar.
OBJECTIVE
To examine the capabilities of a recently developed hand portable GC/IMS instrument, the EVM II, with respect to applicable chemical species, application scenarios, available detection limits & basic separation capabilities.
EXPERIMENTAL CONDITIONS
IMS Conditions: Temperature - 25-100 C, Scan Rate 4 scans/s, Cell Length - 3.9 cm, Reagent - H2O, Voltage - +/- 950 V, Source Flow - 40 ml/min, Gating Pulse - 200 µs, Drift Flow - 160 ml/min, Ion Source - 63Ni, Corona Pressure - 700 mbar
GC Conditions: Phase - DB-1, Column Length - 5 m, Column Diameter - 0.5 mm, Film Thickness - 0.25 µm, Temperature - 25-100 C, Flow Rate - 20 ml/min
AVS Conditions: Pressure (ambient) - 850 mbar, Injection Pulse - 1,2,4,10 s, Carrier Gas - Nitrogen as scrubbed air, Temperature 25-120 C
RESULTS AND DISCUSSION
Figure 1 illustrates the capability of this approach to separate complex mixtures of analytes. A collection of 5 pesticide headspaces (Phorate, Phosdrin, Naled, Meta systox and Dichlorvos) are simultaneously introduced to the instrument. To add to the complexity of the mixture, all 5 pesticides contain additional volatile impurities, so that the separation illustrated in the figure include more than 15 different chemical compounds. (Additional details of pesticide analysis with the EVM II may be found in FemtoScan's Application Report 9701.) This pesticide separation provides one example of the potential for field screening application of the EVM II. In the context of process monitoring, Figure 2 illustrates the detection of menthol at the mid-ppbv level in an (unlit) cigarette headspace. This allows rapid identification and evaluation of menthol cigarettes for process control purposes in a background of other volatile species.
Figure 3 shows the detection of nitroglycerin (NG) in
a headspace above lactose. The analyte concentration is less than the 340
ppbv vapor pressure of NG and includes a separation of the NG from volatile
impurities. This separation illustrates both the connection of the EVM
II to existing IMS application bases for detection of explosives and drugs,
and the potential of the technique as a screening tool for pharmaceuticals
in a process environment. Figure 4 illustrates the detection of acetaldehyde
from a low ppm level vapor standard. This separation was performed using
N2 carrier gas and provides the dry conditions required for the detection
of small somewhat polar, molecular species and non-polar analytes. (This
analysis is not possible with a conventional hand held membrane inlet IMS
instrument.) 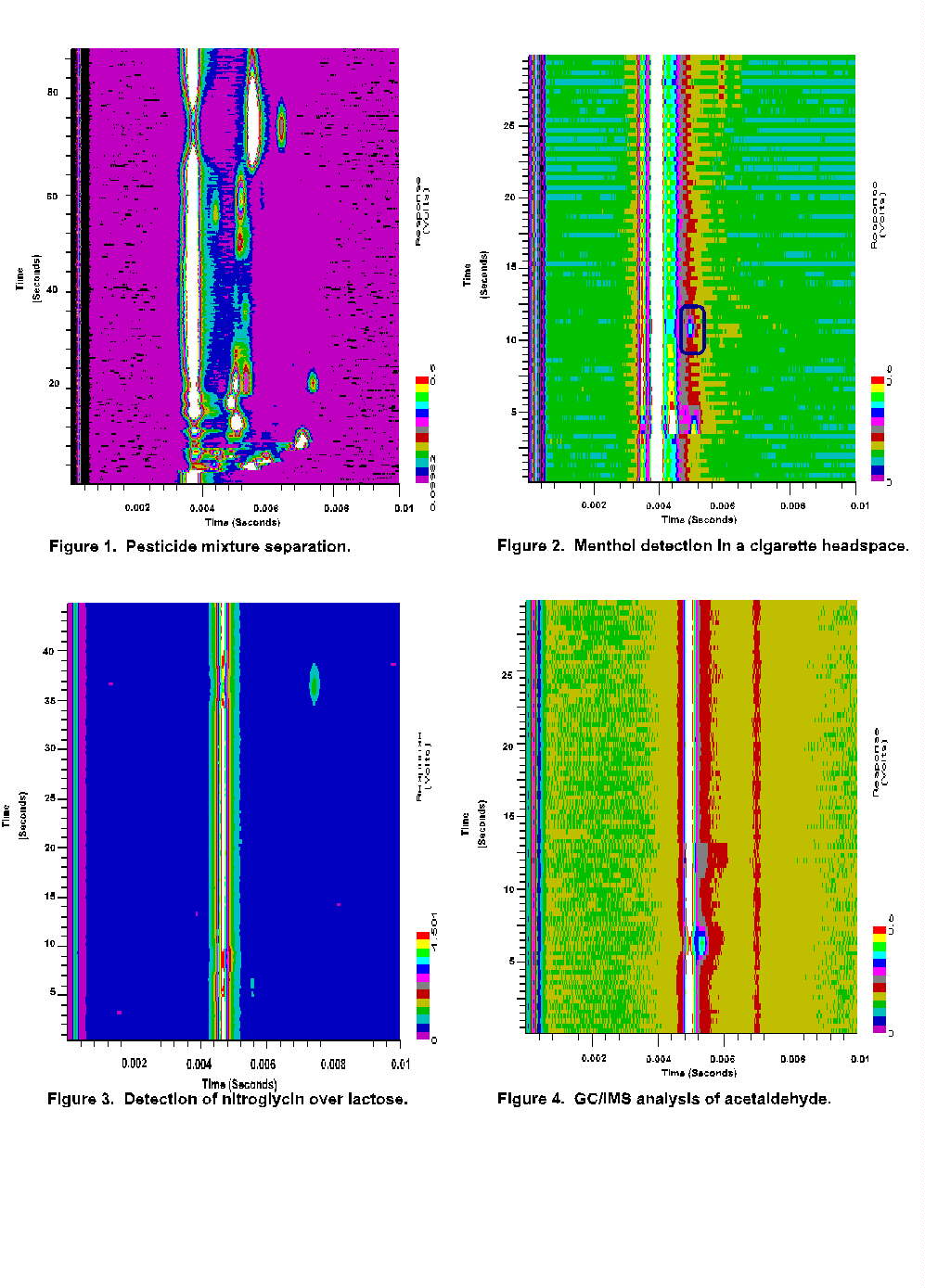
The ability to detect such species at sub ppm levels (see sample response table for acetaldehyde, benzene and hexane) opens up a number of industrial monitoring and environmental applications for the EVM II. The following Sample Analyte Response table lists a number of chemical species suited to detection applications using the EVM II.
Sample EVM II Analyte Responses - (Actual LOD may be Lower)
| Material | Condition | Conc | Material | Condition | Conc | |
| Acetone | 1 | <83 ppb | Hexachlorobenzene* | 3 | HS | |
| Acetonitrile | 1 | 234 ppb | Hexanol | 1 | 49 ppb | |
| Acetophenone | 1 | 52 ppb | 1-Hexene | 2 | 530 ppb | |
| Anisole | 1 | 113 ppb | N-Methylaniline | 1 | 282 ppb | |
| Benzene | 2 | 539 ppb | Methylcyclohexane | 1 | 300 ppm | |
| 1-Butanol | 1 | 134 ppb | Methyl Formamide | 1 | 17 ppb | |
| Iso-Butanol | 1 | 66 ppb | 3-Methyl-2-Hexanone | 1 | 89 ppb | |
| t-Butanol | 1 | 65 ppb | 2-Methylpentane | 1 | 10 ppm | |
| Butyl Acetate | 1 | 46 ppb | Naled | 3 | 1.3 ppm | |
| m-Cresol | 1 | 58 ppb | Nitrobenzene | 1 | 214 ppb | |
| Cumene | 1 | 131 ppb | Octane | 1 | 300 ppm | |
| Cyclopentane | 1 | 100 ppm | 2-Octanol | 1 | 77 ppb | |
| Cyclopentanol | 1 | 337 ppm | Phenethylamine | 1 | 48 ppb | |
| Dichlorvos | 3 | 1.1 ppb | Phenyl Acetonitrile | 1 | 22 ppb | |
| Diethyl Ethyl Phosphonate (DEEP) | 4 | 50 ppb | Phorate | 3 | 390 ppb | |
| Diethyl Methyl Phosphonate (DEMP) | 5 | 7 ppb | Phosdrin | 3 | 150 ppb | |
| N,N-Dimethylaniline | 1 | 96 ppb | 1-Propanol | 1 | 82 ppb | |
| N,N-Dimethyl Formamide | 1 | 334 ppb | Iso-Propenyl Acetate | 1 | 277 ppb | |
| Dimethyl Methyl Phosphonate (DMMP) | 5 | 1 ppb | n-Propyl Ether | 1 | 4.40 ppm | |
| Di-iso-Propyl Methyl Phosphonate (DIMP) | 5 | 4 ppb | m-Toluidine | 1 | 113 ppb | |
| Ethyl Acetate | 1 | 62 ppb | 2,2,4-Trimethylpentane | 1 | 500 ppm | |
| Formaldehyde (37% soln) | 1 | 450 ppb | Valeric Acid | 1 | 56 ppb | |
| Formamide | 1 | 746 ppb | Nitroglycerin | 1 | <340 ppb |
Table Footnotes: (1) - Oven temp-70 C; Inlet Temp -70 C; Cell Temp-70 C, Carrier Gas-Air; (2) - Oven temp-25 C; Inlet Temp -25 C; Cell Temp-25 C, Carrier Gas-N2; (3) - Oven temp-100 C; Inlet Temp -120 C; Cell Temp-100 C, Carrier Gas-N2; (4) - Oven temp-100 C; Inlet Temp -100 C; Cell Temp-100 C, Carrier Gas-Air; (5) - Oven temp-70 C; Inlet Temp -70 C; Cell Temp-70 C, Carrier Gas-N2; * = Negative Ion Mode
CONCLUSIONS
Additional links:
If you have comments or suggestions, please email us at femtoscan.com
Copyright © 1997-1999 FemtoScan Corporation
Page last updated on 7/19/99